Membrane Transport Machineries
Our research comprises the structure/function relationship of membrane transporters involved in efflux.
Bacteria have a plethora of mechanisms to protect themselves against noxious compounds like antibiotics. Many of them possess drug efflux pumps to extrude drugs out of the cell and keep the therapeutic concentration below the toxic threshold so the bacteria will survive. These drug efflux systems play key roles in many multi-resistant pathogens. The phenotype “resistance” is rather complex and entails many cellular mechanisms. Bacteria have many intrinsic resistance mechanisms which may or may not have complete different physiological roles dependent on the environmental setting. Gram-negative bacteria have in addition to their inner membrane – and rather thin-layered peptidoglycan layer – an asymmetric outer membrane, which act as a formidable barrier for some toxic compounds. Those toxins (like many antibiotics) which cross this barrier are often captured by inner membrane embedded efflux pumps. In Gram-negative bacteria, these pumps may be single-component transporters or, in conjunction with a periplasmic adaptor protein and an outer membrane channel, can assemble into more complex systems. These membrane transport machineries export the toxins across the outer membrane as a potent mechanism of resistance.
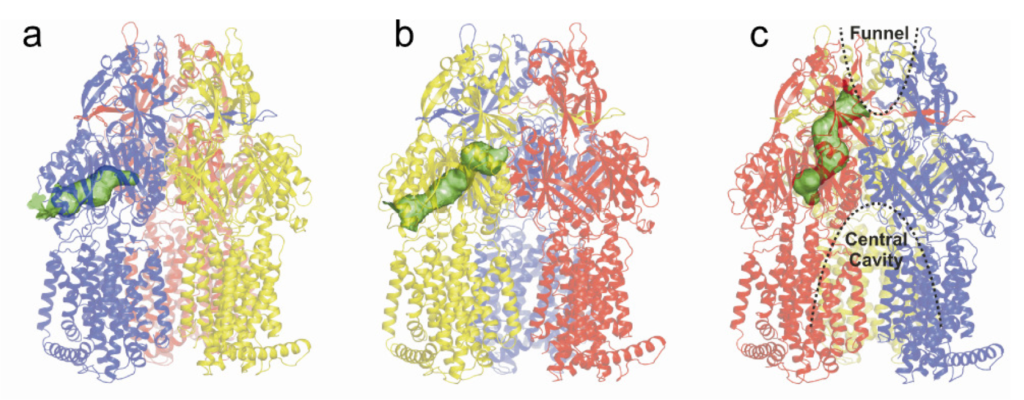
Structures of RND pumps
The complex drug-extruding transporter systems, which are often referred to as “tripartite” efflux pumps, can have a very diverse setup. The inner membrane components are members of the Major Facilitator Superfamily (MFS), the ATP-Binding Cassette (ABC) Superfamily, or the Resistance Nodulation and (cell) Division (RND) superfamily. Members of the latter superfamily are characterized by a wide substrate specificity. To understand how these transporters distinguish between substrate or non-substrate and to understand the nature of inhibitor binding by these transporters, structural (and also functional) insights are necessary. We try to grow crystals of several RND superfamily members, expose the crystals to x-rays, and aim to solve their 3D-structure by crystallography. Another method we use is single-particle Cryo-EM (in collaboration) to obtain structural insights of these transporters in the membrane environment.
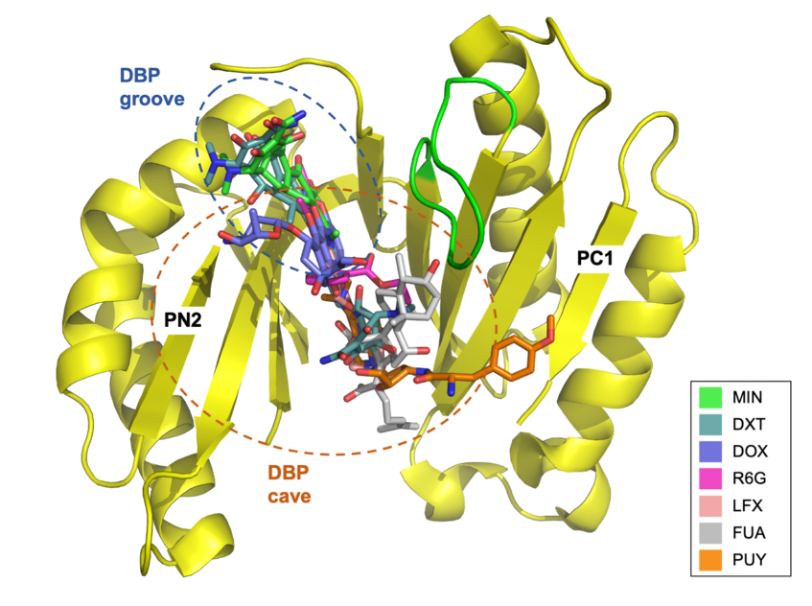
The molecular determinants of polyspecificity
The RND-type drug efflux transporters are characterized by their substrate promiscuity. To understand the molecular determinants of this polyspecificity, we characterize variants of the RND member AcrB from Escherichia coli selected by directed-evolution. Structural and functional insights on substrate binding are obtained using deep sequencing analysis, functional assays, and x-ray crystallography.
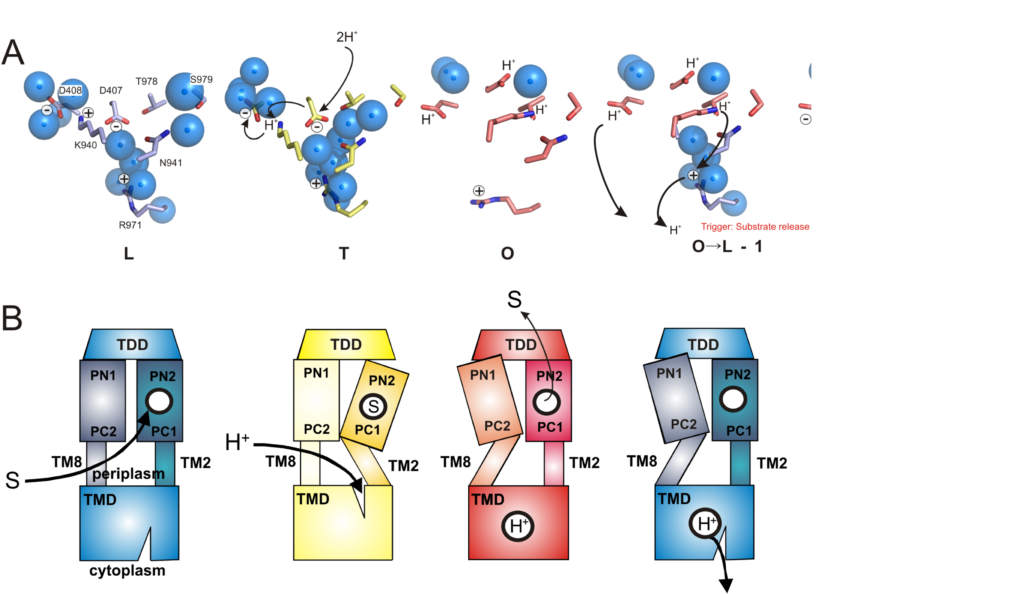
Energy coupling and the transmembrane region
Many bacterial drug efflux transporters are secondary antiporters, i.e. they couple and energize the efflux of drug molecules to a gradient of ions, mostly protons or sometimes sodium ions. Besides the primary ion binding sites in the transmembrane region of these transporters, we also trying to understand how the energy transduction towards the drug binding site occurs (and vice versa). An important aspect is the network of water molecules, which act as a hydrogen bond network facilitating interaction between side chains and the transport of protons. We aim to trap intermediate states of energy transduction (different protein conformations) as to capture different moments of the transport cycle and analyze them via x-ray crystallography.
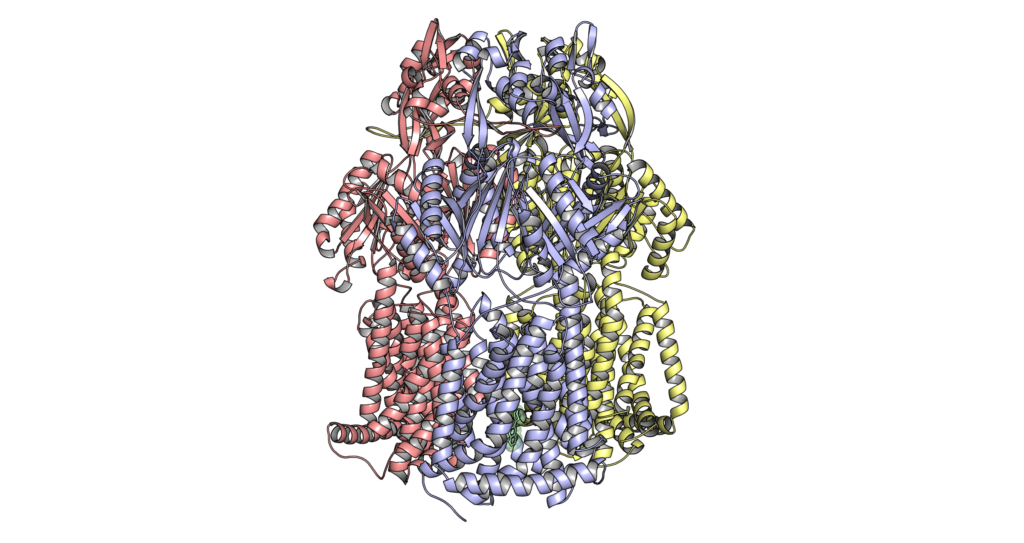
Inhibition of drug efflux pumps
As many clinical isolates overproduce efflux pumps as part of their (multiple) drug resistance phenotype, we are interested to find inhibitors of the pumps. Currently, we are focused on the inhibition of RND-type pumps. In collaboration with Microbiotix, we conduct structure/function analysis on their potent pyranopyridine MBX inhibitors.
Pump efflux networks are part of the efflux resistance phenotype
Amazingly, bacteria deploy different pumps of different classes during antibiotic stress. These pumps often are characterized by an overlapping substrate specificity. Not much is known on how these transporters interact (physically or functionally), but some show a synergistic pump activity. That is why we also do research on the single component transporters of the MFS and SMR family. The final goal will be the co-reconstitution of these transporters in liposomes to directly show the synergistic transport activity.
An overview of these intriguing drug exporting systems is given in a special issue in Research of Microbiology: Drug Efflux Pumps (link).
RND systems, it is not only about drug resistance
Transporters of the RND superfamily are ubiquitous in all domains of life. Whereas the transmembrane region is quite conserved, the soluble domains (periplasmic, extracellular or luminal) are widely different in size and sequence. As we focus on Gram-negative bacteria, we are interested in the synthesis of the outer membrane. It appears that RND transporters (as do ABC transporters) are involved in the lipid homeostasis and trafficking to or from the outer membrane.
Summary
We are fascinated by the molecular mechanisms of membrane transport in general, but those of RND systems in particular. That is why we dig deeply into these proteins itself to analyze the structure/function relationship on the molecular level.
We strongly believe that understanding of resistance mechanisms on the molecular level is necessary to understand resistance per sé.
However, we also believe that an integrative approach i.e. combining the antibiotic resistance research on different levels (i.e. bench to bedside) is absolutely necessary to successfully counteract the problematic of multiple antibiotic resistance.
